We are very excited to welcome a new partner to the TrackMy Solutions team, Dr. Aakash Shah. Dr. Shah is an Orthopaedic Surgeon in Overland Park, Kansas. He is a…

Every year more and more people are getting medical device implants installed in their body. This includes hips, knees, cardiac devices, cataract lenses and more. These devices are amazing life…

Powerhouse Google, is set to collaborate with Fitbit to accelerate digital health and wearables. Check out Fitbit’s press release here.

The amount of technology we have available to us in the world today is astounding. Wherever we go it’s around us, with us, and in many cases, watching us. This…

OWH-Funded Research: Medical Device Safety The FDA Office of Women’s Health awards research grants for 1-2 year studies to support FDA regulatory decision-making and advance the science of women’s health….

FDA has recently approved the following devices to be marketed: PulseRider Aneurysm Neck Reconstruction Device (“PulseRider”) – H160002 The PulseRider is a permanent nitinol (nickel titanium) self-expanding stent implant for…

A thanks to @US_FDA for linking to our website and App download. We’re a part of the #OpenFDA Developer Community. https://buff.ly/2FGVeEq Check it out! #medicaldevice #surgery #medical #FDA
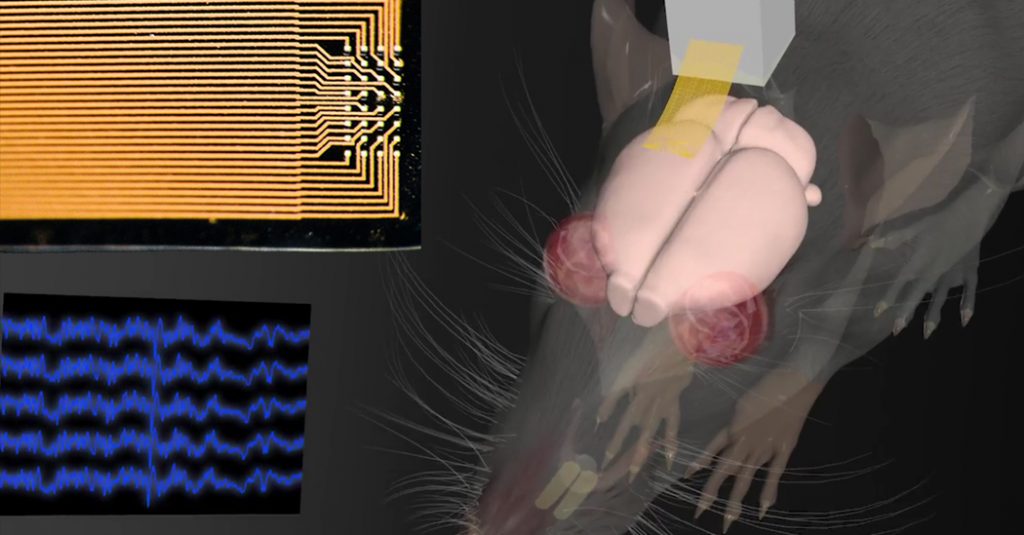
This technology could help clinicians to diagnose and monitor #neurological conditions such as #epilepsy. In the future it may be useful for brain-machine interfaces, such as those controlling #prosthetic limbs….
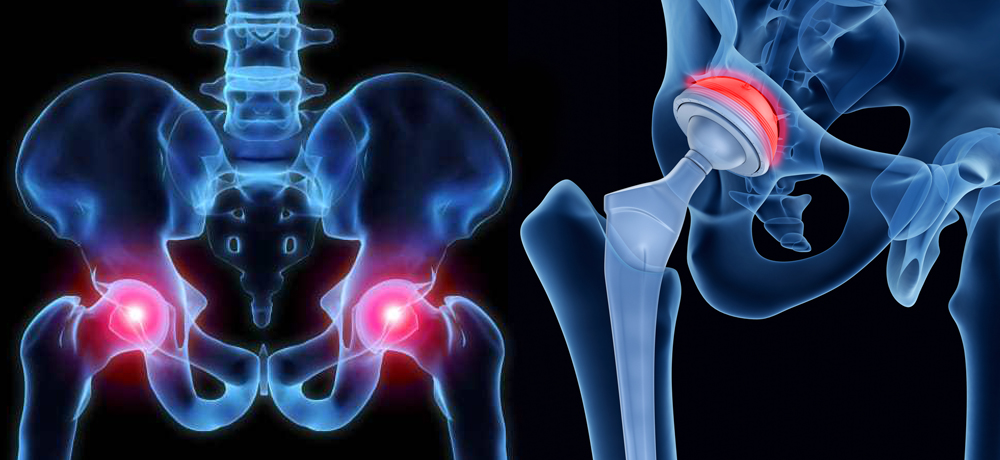
Know about your medical device implant. Here is the recall class breakdown. An FDA “Class I Recall” is the most urgent type of recall that the FDA will issue. In…

Breasts implants with unexplainable symptoms Facing unexplainable symptoms, Kansas City Metro Women argue silicone breast implants made them sick. Read the full story here.
Is the medical device industry moving too fast to keep patients safe? Are much needed clinical tests being skipped? Check out this article by Jeanne Lenzer. #medicaldevice #physicaltherapy Take action…

Do you or someone you know have a medical device implant? Do you love FREE COFFEE? Only two simple steps to be entered for a chance to win. 1.) Download…

Excellent Read Here! FDA Commissioner Scott Gottlieb, M.D., commits to improving the FDA regulated recall process. The current push and ongoing focus of improving this process only further supports our…

UDI stands for Unique Device Identifier. Barcodes aren’t just for the department stores anymore. UDI is becoming a standard topic among healthcare providers. Check out this article by Karen Conway,…

Compliance Dates for UDI Requirements The table below outlines key compliance dates in the UDI final rule. Compliance Date Requirement 1 year after publication of the final rule (September 24,…

